Our Technology
The MSC therapeutic landscape is currently divided between companies favouring the safety of natural MSCs (as demonstrated by almost 30 years of clinical trials), despite the high cost of goods and variable clinical efficacy, and those progressing the more standardized and scalable iPSCs, which address these barriers but are hampered by complex manufacturing and question marks over safety. An innovation elucidated by Prof Anthony Hollander and his research team in the course of a multi-year, painstaking research programme, offers a unique opportunity to develop cell based therapies along a completely novel trajectory, that enables the therapeutic use of natural MSCs with their enviable safety profile in a way which also offers the standardised, scalable benefits of iPSCs.
MSCs occur in a number of tissues but are most commonly isolated from bone marrow and adipose tissue. Their utility in regenerative medicine has historically been based on their tri-lineage differentiation potential (adipogenic, osteogenic and chondrogenic), their immunomodulatory effects and the so-called “trophic effects” of their array of secreted proteins. The differentiation capacity falls off sharply after a small number of doublings in vitro, which has been a major barrier to producing sufficient quantity of cells for standardisation or optimisation purposes. It was assumed that the same was true of the other therapeutic properties of these cells, until the watershed research by Professor Hollander’s group clearly demonstrated the long term persistence in vitro of all of the other restorative properties.
Figure 1 below, showing the falling capacity to differentiate into cartilage with increasing passage number, clearly demonstrates the prevailing orthodoxy that therapeutic function declines rapidly with time in vitro.
Figure 1 Illustrates loss of chondrogenic potential of MSCs with time in vitro from P2-P16
However, in stark contrast to these findings, the ability of cells to stimulate integration and repair of meniscal cartilage over extensive passage numbers is shown in Figure 2 below. This demonstrates that the average percentage of integration is maintained throughout exceptionally high passage numbers, and does not decline in the same way as chondrogenic potential,


Figure 2. The ability to stimulate the integration of meniscal cartilage is retained throughout time in vitro to very high passage numbers.
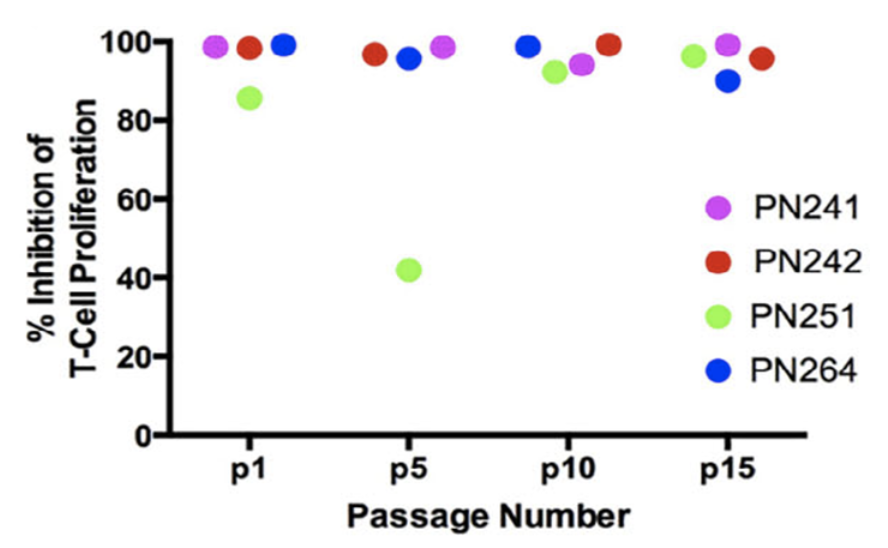
Further research revealed that not only was trophic capacity maintained, but the MSCs also retained their full initial immunomodulatory capacity, as demonstrated by suppression of T Cell proliferation, which is shown in Figure 3 below.
This is a revolutionary innovation – it defines a population of cells which maintain the primary therapeutic attributes of MSCs but are capable of a high level of in vitro expansion. This opens up the possibility of utilising natural MSCs with their enviable safety profile at a previously unimaginable scale of production. In effect, this creates a new therapeutic modality of MSCs – one which is capable of optimisation and standardisation by virtue of the fact that an unprecedented number of therapeutic cells can be derived from a single source.
Specifically, this research identified two key markers which define this MSC population:
- of early passage – the gene for MMP13, a collagenase which, like differentiation potential, decreases as a function of time in vitro and
- of sustained trophic capacity – TIMP1 protein, the most abundant protein in the secretome independent of ageing in vitro.
The definition of an MSC population that is high in TIMP-1 protein and low in MMP13 gene expression therefore describes a novel population of therapeutic MSCs – TrophiCells.
Further Information
Salerno A, Brady K, Rikkers M, Li C, Caamaño-Gutierrez E, Falciani F, Blom AW, Whitehouse MR, Hollander AP. MMP13 and TIMP1 are functional markers for two different potential modes of action by mesenchymal stem/stromal cells when treating osteoarthritis. Stem Cells. 2020 Nov;38(11):1438-1453. doi: 10.1002/stem.3255. Epub 2020 Jul 17. PMID: 32652878.
Patent Estate
| Title | Application Number/s |
|---|---|
| Proliferating Stem Cells | 2305070.1 - UK |
| Injectable stem cell therapy for osteoarthritis | 1805306.6 - UK |
| 1817486.2 - UK | |
| PCT/GB2019/050917 - PCT | |
| 19716520.2 - EPO | |
| 17/042,682 - USA | |
| Use of In vitro aged undifferentiated mesenchymal stem cells to drive healing of tissue damage | 1914235.5 - UK |
| PCT/GB2020/052439 - PCT | |
| 17/761,565 - USA | |
| 20788864.5 - EPO | |
| 2022-520605 - Japan | |
| 2020361080 - Australia | |
| 10-2022-7014379 – South Korea |
The Applicant for all of these is the University of Liverpool.